BiologicGlass™ Granules
Synthetic and bioactive bone graft substitute
BiologicGlass™ Granules is indicated in regenerative medicine as a synthetic bioactive bone substitute which comes in the shape of granules. BiologicGlass™ combines natural biological action and optimum safety for reliable and reproducible results.
Based on innovative technology, BiologicGlass™ is a bioactive glass 45S5 ceramic composed of silicon, sodium, phosphorous and calcium oxides. All of these elements are naturally present in the human body and are key components in the osteogenesis process. This bioactivity property, marks a new stage in bone regeneration products.
The formulation of BiologicGlass™ brings excellent biocompatibility level and drastically reduce the risk of pathogen agents transmission.
BiologicGlass™ technology is fully developed in France with local partners to ensure highest quality.
BiologicGlass™ offers surgeons and patients optimum safety during implantation for a natural and effective bone restoration, for all indications encountered in their daily practice.

BiologicGlass™, Bone graft substitute is a medical device class III, manufactured by NORAKER® and whose conformity assessment was conducted by LNE / G-MED (0459).
BiologicGlass™ is indicated for filling bone defects. Read the instructions supplied with the product for complete information on indications, cons-indications, warnings and precautions, and adverse effects.
Bioactive Glass : the key technology to regenerate your bones
Intended use
BiologicGlass™ Granules is a synthetic, bioactive and absorbable bone substitute, intended for the filling, reconstruction and/or fusion of bone defects or gaps in the skeletal system, in cranio maxillo facial and otorhinolaryngology surgery.

BiologicGlass™ Granules has to be mixed normal saline solution, autologous bone, patient blood or PRF (Platelet-Rich Fibrin). In order to enhance the host site vascularization, heighten the host cortical bone walls. When placing the graft material, pack it with a compress moistened by normal saline solution. Excess filling is recommended.
Indications and target population
Loss or lack of bone substance for bone defects of traumatic, pathological or surgical origin when autologous solutions are not applicable or sufficient in cranio maxillo facial and otorhinolaryngology surgery in children and adult population:
- Filling after surgical bone defect (donor sites after removal of autograft, trepanation, …)
- Filling after removal of cholesteatoma
- Filling and reconstruction due to maxilla and periodontium pathologies (in adult only).
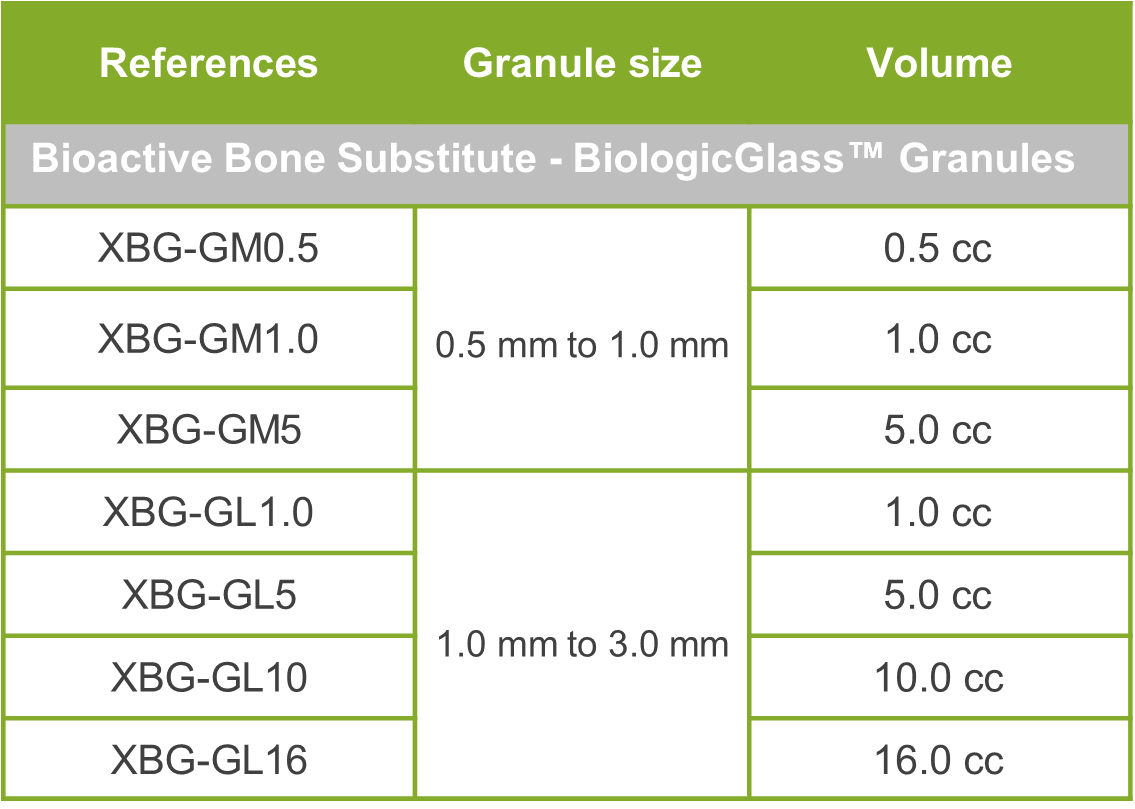
Available References